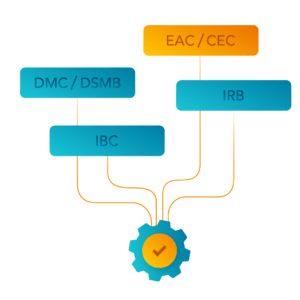
Independent Review Committees for Safety and Compliance
Clinical research involving human participants typically involves some type of risk. Incorporating the appropriate ethics reviews and safety oversight helps ensure stakeholder safety, data integrity, and regulatory compliance.
Safety and Compliance Reviews
-
Institutional Review Board (IRB)
IRBs help ensure the proper protection of clinical trial participants’ rights and welfare.
Federal regulations require an independent group of scientific and non-scientific members review and monitor clinical trials involving human participants.
IRBs review study materials (e.g., protocol, informed consent form [ICF]), investigator credentials, changes in research, and safety events to ensure risks are appropriately minimized and participants are properly informed.
Generally, any materials a potential participant will see regarding the trial should receive an IRB review.
-
Institutional Biosafety Committee (IBC)
IBCs review research involving cell and gene therapy techniques (e.g., engineered genetic material) to ensure the safety of research participants, study staff, and the community in which the study takes place.
IBC oversight is required when either the site or sponsor has ever received National Institutes of Health (NIH) support for recombinant DNA (rDNA) or synthetic nucleic acid (sNA) research.
Even when NIH support is not involved, IBC review is considered an industry best practice.
IBCs perform a biosafety risk assessment to evaluate risks and occupational environmental safety concerns associated with genetic modifications and investigational procedures.
-
Data Safety Monitoring Board (DSMB) / Data Monitoring Committee (DMC)
According to U.S. Food and Drug Administration (FDA) regulation and GCP E6 R2, sponsors must have adequate data safety monitoring plans in their clinical protocols. An independent DSMB satisfies that requirement, providing periodic review of accumulated data during a clinical trial.
DSMB members assess study data to identify any early evidence of benefit or harm, focusing on participant safety and data integrity and validity.
The DSMB may recommend a sponsor modify a trial or halt study activities based on data trends.
As a general rule, if the study is blinded, it probably needs DSMB oversight.
-
Endpoint Adjudication Committee (EAC) / Clinical Event Committee (CEC)
Clinical events taking place during a study must be assessed to determine whether the investigational therapy or other outside factors caused them.
Researchers may also require specialized medical judgment to determine whether a participant has met a protocol-defined endpoint.
These assessments often require additional, independent therapeutic expertise in the form of a CEC or EAC.
Such committees are formed for a specific study, with appropriate medical and clinical expertise to assess the events.

Independent Clinical Trial Safety Oversight
Independence is key to ensuring a committee’s review is unbiased and not influenced by those involved in study conduct.
Few would argue over the importance of IRB review being independent of study priorities, as this allows reviewers to focus solely on participant protections.
Independence also removes the potential for perceived bias, making the review more trustworthy in the eyes of regulators and the public.
It’s similarly critical for other research review committees, like DSMBs and endpoint adjudication committees, to be completely independent of those sponsoring or conducting the study.
Tips for More Efficient Research Reviews
- Communicate your timelines and special requirements to the committee(s) as early as possible
- Work with a single partner who can provide all of the reviews your study requires
- Avoid extended follow-up questions by ensuring you provide all necessary materials in your initial submission
- Communicate your timelines and special requirements to the committee(s) as early as possible
- Ask questions and provide clarifications to ensure the committee understands your position
- Use the committee’s tracking platform or other available technology to stay informed on review activities (and consider integrating your trial technology with the committee’s)
- Request study metrics to understand bottlenecks with the committee and within your organization
- Utilize platforms like SMART IRB to streamline reliance agreement activities

Extensive Reach and Trusted Expertise
institutions, academic medical centers, and hospital systems supported by Advarra’s IRB – more than any other IRB
sites worldwide registered with Advarra’s IBC – more than any other IBC
biostatisticians, medical experts, and clinical professionals globally supporting Advarra’s DSMB and EAC
Advarra’s reviews support 100% of:
-
Top 50 global biopharma sponsors
-
Top 20 CROs
-
NCI-designated cancer centers
Comprehensive Review Solutions for Every Need
Read our Related Resources
Clinical Endpoint Adjudication Committees: Not Just for “Endpoints”
This blog outlines critical events where EACs are used to look at more than just “endpoints” in clinical research.
Reporting to the IRB: What Does and Does Not Need to be Reported
This white paper outlines IRB reporting requirements, why reporting is important, and what should and should not be reported.
Does This Study Require IBC Review?
While clinical research professionals are familiar with the role of the IRB to protect research subjects, most are unfamiliar with the added need for an Institutional Biosafety Committee (IBC).